2024
Sattari, Kianoosh; Li, Dawei; Kalita, Bhupalee; Xie, Yunchao; Lighvan, Fatemeh Barmaleki; Isayev, Olexandr; Lin, Jian
De novo molecule design towards biased properties via a deep generative framework and iterative transfer learning Journal Article
In: Digital Discovery, vol. 3, no. 2, pp. 410–421, 2024.
Abstract | Links | BibTeX | Tags: Active learning, Generative AI
@article{Sattari2024,
title = {\textit{De novo} molecule design towards biased properties \textit{via} a deep generative framework and iterative transfer learning},
author = {Kianoosh Sattari and Dawei Li and Bhupalee Kalita and Yunchao Xie and Fatemeh Barmaleki Lighvan and Olexandr Isayev and Jian Lin},
doi = {10.1039/d3dd00210a},
year = {2024},
date = {2024-02-14},
urldate = {2024-02-14},
journal = {Digital Discovery},
volume = {3},
number = {2},
pages = {410--421},
publisher = {Royal Society of Chemistry (RSC)},
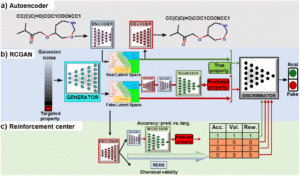
abstract = {De novo design of molecules with targeted properties represents a new frontier in molecule development. Despite enormous progress, two main challenges remain: (i) generating novel molecules conditioned on targeted, continuous property values; (ii) obtaining molecules with property values beyond the range in the training data. To tackle these challenges, we propose a reinforced regressional and conditional generative adversarial network (RRCGAN) to generate chemically valid molecules with targeted HOMO\textendashLUMO energy gap (ΔEH\textendashL) as a proof-of-concept study. As validated by density functional theory (DFT) calculation, 75% of the generated molecules have a relative error (RE) of \<20% of the targeted ΔEH\textendashL values. To bias the generation toward the ΔEH\textendashL values beyond the range of the original training molecules, transfer learning was applied to iteratively retrain the RRCGAN model. After just two iterations, the mean ΔEH\textendashL of the generated molecules increases to 8.7 eV from the mean value of 5.9 eV shown in the initial training dataset. Qualitative and quantitative analyses reveal that the model has successfully captured the underlying structure\textendashproperty relationship, which agrees well with the established physical and chemical rules. These results present a trustworthy, purely data-driven methodology for the highly efficient generation of novel molecules with different targeted properties.},
keywords = {Active learning, Generative AI},
pubstate = {published},
tppubtype = {article}
}
Tropsha, Alexander; Isayev, Olexandr; Varnek, Alexandre; Schneider, Gisbert; Cherkasov, Artem
Integrating QSAR modelling and deep learning in drug discovery: the emergence of deep QSAR Journal Article
In: Nat Rev Drug Discov, vol. 23, no. 2, pp. 141–155, 2024.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, Review
@article{Tropsha2023,
title = {Integrating QSAR modelling and deep learning in drug discovery: the emergence of deep QSAR},
author = {Alexander Tropsha and Olexandr Isayev and Alexandre Varnek and Gisbert Schneider and Artem Cherkasov},
doi = {10.1038/s41573-023-00832-0},
year = {2024},
date = {2024-01-12},
urldate = {2024-01-12},
journal = {Nat Rev Drug Discov},
volume = {23},
number = {2},
pages = {141--155},
publisher = {Springer Science and Business Media LLC},
abstract = {Quantitative structure\textendashactivity relationship (QSAR) modelling, an approach that was introduced 60 years ago, is widely used in computer-aided drug design. In recent years, progress in artificial intelligence techniques, such as deep learning, the rapid growth of databases of molecules for virtual screening and dramatic improvements in computational power have supported the emergence of a new field of QSAR applications that we term ‘deep QSAR’. Marking a decade from the pioneering applications of deep QSAR to tasks involved in small-molecule drug discovery, we herein describe key advances in the field, including deep generative and reinforcement learning approaches in molecular design, deep learning models for synthetic planning and the application of deep QSAR models in structure-based virtual screening. We also reflect on the emergence of quantum computing, which promises to further accelerate deep QSAR applications and the need for open-source and democratized resources to support computer-aided drug design.},
keywords = {Drug Discovery, Generative AI, Review},
pubstate = {published},
tppubtype = {article}
}
2023

Anstine, Dylan M.; Isayev, Olexandr
Generative Models as an Emerging Paradigm in the Chemical Sciences Journal Article
In: J. Am. Chem. Soc., vol. 145, no. 16, pp. 8736–8750, 2023.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, Review, RL
@article{Anstine2023b,
title = {Generative Models as an Emerging Paradigm in the Chemical Sciences},
author = {Dylan M. Anstine and Olexandr Isayev},
doi = {10.1021/jacs.2c13467},
year = {2023},
date = {2023-04-26},
urldate = {2023-04-26},
journal = {J. Am. Chem. Soc.},
volume = {145},
number = {16},
pages = {8736--8750},
publisher = {American Chemical Society (ACS)},
abstract = {Traditional computational approaches to design chemical species are limited by the need to compute properties for a vast number of candidates, e.g., by discriminative modeling. Therefore, inverse design methods aim to start from the desired property and optimize a corresponding chemical structure. From a machine learning viewpoint, the inverse design problem can be addressed through so-called generative modeling. Mathematically, discriminative models are defined by learning the probability distribution function of properties given the molecular or material structure. In contrast, a generative model seeks to exploit the joint probability of a chemical species with target characteristics. The overarching idea of generative modeling is to implement a system that produces novel compounds that are expected to have a desired set of chemical features, effectively sidestepping issues found in the forward design process. In this contribution, we overview and critically analyze popular generative algorithms like generative adversarial networks, variational autoencoders, flow, and diffusion models. We highlight key differences between each of the models, provide insights into recent success stories, and discuss outstanding challenges for realizing generative modeling discovered solutions in chemical applications.},
keywords = {Drug Discovery, Generative AI, Review, RL},
pubstate = {published},
tppubtype = {article}
}
2022
Korshunova, Maria; Huang, Niles; Capuzzi, Stephen; Radchenko, Dmytro S.; Savych, Olena; Moroz, Yuriy S.; Wells, Carrow I.; Willson, Timothy M.; Tropsha, Alexander; Isayev, Olexandr
Generative and reinforcement learning approaches for the automated de novo design of bioactive compounds Journal Article
In: Commun Chem, vol. 5, no. 1, pp. 129 , 2022.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, RL
@article{Korshunova2022,
title = {Generative and reinforcement learning approaches for the automated de novo design of bioactive compounds},
author = {Maria Korshunova and Niles Huang and Stephen Capuzzi and Dmytro S. Radchenko and Olena Savych and Yuriy S. Moroz and Carrow I. Wells and Timothy M. Willson and Alexander Tropsha and Olexandr Isayev},
doi = {10.1038/s42004-022-00733-0},
year = {2022},
date = {2022-03-31},
urldate = {2022-03-31},
journal = {Commun Chem},
volume = {5},
number = {1},
pages = {129 },
publisher = {Springer Science and Business Media LLC},
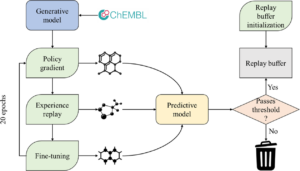
abstract = {\<jats:title\>Abstract\</jats:title\>\<jats:p\>Deep generative neural networks have been used increasingly in computational chemistry for \<jats:italic\>de novo\</jats:italic\> design of molecules with desired properties. Many deep learning approaches employ reinforcement learning for optimizing the target properties of the generated molecules. However, the success of this approach is often hampered by the problem of sparse rewards as the majority of the generated molecules are expectedly predicted as inactives. We propose several technical innovations to address this problem and improve the balance between exploration and exploitation modes in reinforcement learning. In a proof-of-concept study, we demonstrate the application of the deep generative recurrent neural network architecture enhanced by several proposed technical tricks to design inhibitors of the epidermal growth factor (EGFR) and further experimentally validate their potency. The proposed technical solutions are expected to substantially improve the success rate of finding novel bioactive compounds for specific biological targets using generative and reinforcement learning approaches.\</jats:p\>},
keywords = {Drug Discovery, Generative AI, RL},
pubstate = {published},
tppubtype = {article}
}