2023

Anstine, Dylan M.; Isayev, Olexandr
Generative Models as an Emerging Paradigm in the Chemical Sciences Journal Article
In: J. Am. Chem. Soc., vol. 145, no. 16, pp. 8736–8750, 2023.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, Review, RL
@article{Anstine2023b,
title = {Generative Models as an Emerging Paradigm in the Chemical Sciences},
author = {Dylan M. Anstine and Olexandr Isayev},
doi = {10.1021/jacs.2c13467},
year = {2023},
date = {2023-04-26},
urldate = {2023-04-26},
journal = {J. Am. Chem. Soc.},
volume = {145},
number = {16},
pages = {8736--8750},
publisher = {American Chemical Society (ACS)},
abstract = {Traditional computational approaches to design chemical species are limited by the need to compute properties for a vast number of candidates, e.g., by discriminative modeling. Therefore, inverse design methods aim to start from the desired property and optimize a corresponding chemical structure. From a machine learning viewpoint, the inverse design problem can be addressed through so-called generative modeling. Mathematically, discriminative models are defined by learning the probability distribution function of properties given the molecular or material structure. In contrast, a generative model seeks to exploit the joint probability of a chemical species with target characteristics. The overarching idea of generative modeling is to implement a system that produces novel compounds that are expected to have a desired set of chemical features, effectively sidestepping issues found in the forward design process. In this contribution, we overview and critically analyze popular generative algorithms like generative adversarial networks, variational autoencoders, flow, and diffusion models. We highlight key differences between each of the models, provide insights into recent success stories, and discuss outstanding challenges for realizing generative modeling discovered solutions in chemical applications.},
keywords = {Drug Discovery, Generative AI, Review, RL},
pubstate = {published},
tppubtype = {article}
}
Traditional computational approaches to design chemical species are limited by the need to compute properties for a vast number of candidates, e.g., by discriminative modeling. Therefore, inverse design methods aim to start from the desired property and optimize a corresponding chemical structure. From a machine learning viewpoint, the inverse design problem can be addressed through so-called generative modeling. Mathematically, discriminative models are defined by learning the probability distribution function of properties given the molecular or material structure. In contrast, a generative model seeks to exploit the joint probability of a chemical species with target characteristics. The overarching idea of generative modeling is to implement a system that produces novel compounds that are expected to have a desired set of chemical features, effectively sidestepping issues found in the forward design process. In this contribution, we overview and critically analyze popular generative algorithms like generative adversarial networks, variational autoencoders, flow, and diffusion models. We highlight key differences between each of the models, provide insights into recent success stories, and discuss outstanding challenges for realizing generative modeling discovered solutions in chemical applications.
2022
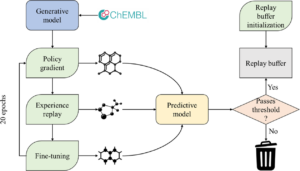
Korshunova, Maria; Huang, Niles; Capuzzi, Stephen; Radchenko, Dmytro S.; Savych, Olena; Moroz, Yuriy S.; Wells, Carrow I.; Willson, Timothy M.; Tropsha, Alexander; Isayev, Olexandr
Generative and reinforcement learning approaches for the automated de novo design of bioactive compounds Journal Article
In: Commun Chem, vol. 5, no. 1, pp. 129 , 2022.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, RL
@article{Korshunova2022,
title = {Generative and reinforcement learning approaches for the automated de novo design of bioactive compounds},
author = {Maria Korshunova and Niles Huang and Stephen Capuzzi and Dmytro S. Radchenko and Olena Savych and Yuriy S. Moroz and Carrow I. Wells and Timothy M. Willson and Alexander Tropsha and Olexandr Isayev},
doi = {10.1038/s42004-022-00733-0},
year = {2022},
date = {2022-03-31},
urldate = {2022-03-31},
journal = {Commun Chem},
volume = {5},
number = {1},
pages = {129 },
publisher = {Springer Science and Business Media LLC},
abstract = {\<jats:title\>Abstract\</jats:title\>\<jats:p\>Deep generative neural networks have been used increasingly in computational chemistry for \<jats:italic\>de novo\</jats:italic\> design of molecules with desired properties. Many deep learning approaches employ reinforcement learning for optimizing the target properties of the generated molecules. However, the success of this approach is often hampered by the problem of sparse rewards as the majority of the generated molecules are expectedly predicted as inactives. We propose several technical innovations to address this problem and improve the balance between exploration and exploitation modes in reinforcement learning. In a proof-of-concept study, we demonstrate the application of the deep generative recurrent neural network architecture enhanced by several proposed technical tricks to design inhibitors of the epidermal growth factor (EGFR) and further experimentally validate their potency. The proposed technical solutions are expected to substantially improve the success rate of finding novel bioactive compounds for specific biological targets using generative and reinforcement learning approaches.\</jats:p\>},
keywords = {Drug Discovery, Generative AI, RL},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>Deep generative neural networks have been used increasingly in computational chemistry for <jats:italic>de novo</jats:italic> design of molecules with desired properties. Many deep learning approaches employ reinforcement learning for optimizing the target properties of the generated molecules. However, the success of this approach is often hampered by the problem of sparse rewards as the majority of the generated molecules are expectedly predicted as inactives. We propose several technical innovations to address this problem and improve the balance between exploration and exploitation modes in reinforcement learning. In a proof-of-concept study, we demonstrate the application of the deep generative recurrent neural network architecture enhanced by several proposed technical tricks to design inhibitors of the epidermal growth factor (EGFR) and further experimentally validate their potency. The proposed technical solutions are expected to substantially improve the success rate of finding novel bioactive compounds for specific biological targets using generative and reinforcement learning approaches.</jats:p>