2024
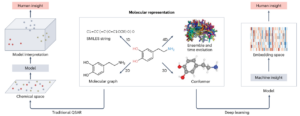
Tropsha, Alexander; Isayev, Olexandr; Varnek, Alexandre; Schneider, Gisbert; Cherkasov, Artem
Integrating QSAR modelling and deep learning in drug discovery: the emergence of deep QSAR Journal Article
In: Nat Rev Drug Discov, vol. 23, no. 2, pp. 141–155, 2024.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, Review
@article{Tropsha2023,
title = {Integrating QSAR modelling and deep learning in drug discovery: the emergence of deep QSAR},
author = {Alexander Tropsha and Olexandr Isayev and Alexandre Varnek and Gisbert Schneider and Artem Cherkasov},
doi = {10.1038/s41573-023-00832-0},
year = {2024},
date = {2024-01-12},
urldate = {2024-01-12},
journal = {Nat Rev Drug Discov},
volume = {23},
number = {2},
pages = {141--155},
publisher = {Springer Science and Business Media LLC},
abstract = {Quantitative structure\textendashactivity relationship (QSAR) modelling, an approach that was introduced 60 years ago, is widely used in computer-aided drug design. In recent years, progress in artificial intelligence techniques, such as deep learning, the rapid growth of databases of molecules for virtual screening and dramatic improvements in computational power have supported the emergence of a new field of QSAR applications that we term ‘deep QSAR’. Marking a decade from the pioneering applications of deep QSAR to tasks involved in small-molecule drug discovery, we herein describe key advances in the field, including deep generative and reinforcement learning approaches in molecular design, deep learning models for synthetic planning and the application of deep QSAR models in structure-based virtual screening. We also reflect on the emergence of quantum computing, which promises to further accelerate deep QSAR applications and the need for open-source and democratized resources to support computer-aided drug design.},
keywords = {Drug Discovery, Generative AI, Review},
pubstate = {published},
tppubtype = {article}
}
2023

Anstine, Dylan M.; Isayev, Olexandr
Generative Models as an Emerging Paradigm in the Chemical Sciences Journal Article
In: J. Am. Chem. Soc., vol. 145, no. 16, pp. 8736–8750, 2023.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, Review, RL
@article{Anstine2023b,
title = {Generative Models as an Emerging Paradigm in the Chemical Sciences},
author = {Dylan M. Anstine and Olexandr Isayev},
doi = {10.1021/jacs.2c13467},
year = {2023},
date = {2023-04-26},
urldate = {2023-04-26},
journal = {J. Am. Chem. Soc.},
volume = {145},
number = {16},
pages = {8736--8750},
publisher = {American Chemical Society (ACS)},
abstract = {Traditional computational approaches to design chemical species are limited by the need to compute properties for a vast number of candidates, e.g., by discriminative modeling. Therefore, inverse design methods aim to start from the desired property and optimize a corresponding chemical structure. From a machine learning viewpoint, the inverse design problem can be addressed through so-called generative modeling. Mathematically, discriminative models are defined by learning the probability distribution function of properties given the molecular or material structure. In contrast, a generative model seeks to exploit the joint probability of a chemical species with target characteristics. The overarching idea of generative modeling is to implement a system that produces novel compounds that are expected to have a desired set of chemical features, effectively sidestepping issues found in the forward design process. In this contribution, we overview and critically analyze popular generative algorithms like generative adversarial networks, variational autoencoders, flow, and diffusion models. We highlight key differences between each of the models, provide insights into recent success stories, and discuss outstanding challenges for realizing generative modeling discovered solutions in chemical applications.},
keywords = {Drug Discovery, Generative AI, Review, RL},
pubstate = {published},
tppubtype = {article}
}
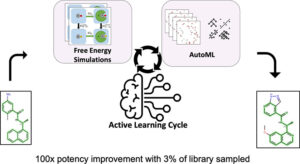
Gusev, Filipp; Gutkin, Evgeny; Kurnikova, Maria G.; Isayev, Olexandr
Active Learning Guided Drug Design Lead Optimization Based on Relative Binding Free Energy Modeling Journal Article
In: J. Chem. Inf. Model., vol. 63, no. 2, pp. 583–594, 2023.
Abstract | Links | BibTeX | Tags: Active learning, Drug Discovery
@article{Gusev2023,
title = {Active Learning Guided Drug Design Lead Optimization Based on Relative Binding Free Energy Modeling},
author = {Filipp Gusev and Evgeny Gutkin and Maria G. Kurnikova and Olexandr Isayev},
doi = {10.1021/acs.jcim.2c01052},
year = {2023},
date = {2023-01-23},
urldate = {2023-01-23},
journal = {J. Chem. Inf. Model.},
volume = {63},
number = {2},
pages = {583--594},
publisher = {American Chemical Society (ACS)},
abstract = {In silico identification of potent protein inhibitors commonly requires prediction of a ligand binding free energy (BFE). Thermodynamics integration (TI) based on molecular dynamics (MD) simulations is a BFE calculation method capable of acquiring accurate BFE, but it is computationally expensive and time-consuming. In this work, we have developed an efficient automated workflow for identifying compounds with the lowest BFE among thousands of congeneric ligands, which requires only hundreds of TI calculations. Automated machine learning (AutoML) orchestrated by active learning (AL) in an AL\textendashAutoML workflow allows unbiased and efficient search for a small set of best-performing molecules. We have applied this workflow to select inhibitors of the SARS-CoV-2 papain-like protease and were able to find 133 compounds with improved binding affinity, including 16 compounds with better than 100-fold binding affinity improvement. We obtained a hit rate that outperforms that expected of traditional expert medicinal chemist-guided campaigns. Thus, we demonstrate that the combination of AL and AutoML with free energy simulations provides at least 20× speedup relative to the na\"{i}ve brute force approaches.},
keywords = {Active learning, Drug Discovery},
pubstate = {published},
tppubtype = {article}
}
2022
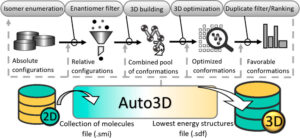
Liu, Zhen; Zubatiuk, Tetiana; Roitberg, Adrian; Isayev, Olexandr
Auto3D: Automatic Generation of the Low-Energy 3D Structures with ANI Neural Network Potentials Journal Article
In: J. Chem. Inf. Model., vol. 62, no. 22, pp. 5373–5382, 2022.
Abstract | Links | BibTeX | Tags: ANI, Drug Discovery
@article{Liu2022,
title = {Auto3D: Automatic Generation of the Low-Energy 3D Structures with ANI Neural Network Potentials},
author = {Zhen Liu and Tetiana Zubatiuk and Adrian Roitberg and Olexandr Isayev},
doi = {10.1021/acs.jcim.2c00817},
year = {2022},
date = {2022-11-28},
urldate = {2022-11-28},
journal = {J. Chem. Inf. Model.},
volume = {62},
number = {22},
pages = {5373--5382},
publisher = {American Chemical Society (ACS)},
abstract = {Computational programs accelerate the chemical discovery processes but often need proper three-dimensional molecular information as part of the input. Getting optimal molecular structures is challenging because it requires enumerating and optimizing a huge space of stereoisomers and conformers. We developed the Python-based Auto3D package for generating the low-energy 3D structures using SMILES as the input. Auto3D is based on state-of-the-art algorithms and can automatize the isomer enumeration and duplicate filtering process, 3D building process, geometry optimization, and ranking process. Tested on 50 molecules with multiple unspecified stereocenters, Auto3D is guaranteed to find the stereoconfiguration that yields the lowest-energy conformer. With Auto3D, we provide an extension of the ANI model. The new model, dubbed ANI-2xt, is trained on a tautomer-rich data set. ANI-2xt is benchmarked with DFT methods on geometry optimization and electronic and Gibbs free energy calculations. Compared with ANI-2x, ANI-2xt provides a 42% error reduction for tautomeric reaction energy calculations when using the gold-standard coupled-cluster calculation as the reference. ANI-2xt can accurately predict the energies and is several orders of magnitude faster than DFT methods.},
keywords = {ANI, Drug Discovery},
pubstate = {published},
tppubtype = {article}
}
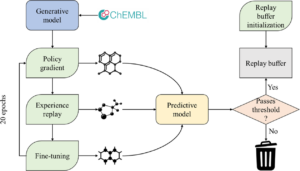
Korshunova, Maria; Huang, Niles; Capuzzi, Stephen; Radchenko, Dmytro S.; Savych, Olena; Moroz, Yuriy S.; Wells, Carrow I.; Willson, Timothy M.; Tropsha, Alexander; Isayev, Olexandr
Generative and reinforcement learning approaches for the automated de novo design of bioactive compounds Journal Article
In: Commun Chem, vol. 5, no. 1, pp. 129 , 2022.
Abstract | Links | BibTeX | Tags: Drug Discovery, Generative AI, RL
@article{Korshunova2022,
title = {Generative and reinforcement learning approaches for the automated de novo design of bioactive compounds},
author = {Maria Korshunova and Niles Huang and Stephen Capuzzi and Dmytro S. Radchenko and Olena Savych and Yuriy S. Moroz and Carrow I. Wells and Timothy M. Willson and Alexander Tropsha and Olexandr Isayev},
doi = {10.1038/s42004-022-00733-0},
year = {2022},
date = {2022-03-31},
urldate = {2022-03-31},
journal = {Commun Chem},
volume = {5},
number = {1},
pages = {129 },
publisher = {Springer Science and Business Media LLC},
abstract = {\<jats:title\>Abstract\</jats:title\>\<jats:p\>Deep generative neural networks have been used increasingly in computational chemistry for \<jats:italic\>de novo\</jats:italic\> design of molecules with desired properties. Many deep learning approaches employ reinforcement learning for optimizing the target properties of the generated molecules. However, the success of this approach is often hampered by the problem of sparse rewards as the majority of the generated molecules are expectedly predicted as inactives. We propose several technical innovations to address this problem and improve the balance between exploration and exploitation modes in reinforcement learning. In a proof-of-concept study, we demonstrate the application of the deep generative recurrent neural network architecture enhanced by several proposed technical tricks to design inhibitors of the epidermal growth factor (EGFR) and further experimentally validate their potency. The proposed technical solutions are expected to substantially improve the success rate of finding novel bioactive compounds for specific biological targets using generative and reinforcement learning approaches.\</jats:p\>},
keywords = {Drug Discovery, Generative AI, RL},
pubstate = {published},
tppubtype = {article}
}
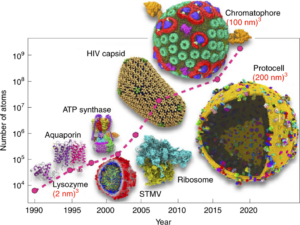
Pandey, Mohit; Fernandez, Michael; Gentile, Francesco; Isayev, Olexandr; Tropsha, Alexander; Stern, Abraham C.; Cherkasov, Artem
The transformational role of GPU computing and deep learning in drug discovery Journal Article
In: Nat Mach Intell, vol. 4, no. 3, pp. 211–221, 2022.
Abstract | Links | BibTeX | Tags: Drug Discovery, Review
@article{Pandey2022,
title = {The transformational role of GPU computing and deep learning in drug discovery},
author = {Mohit Pandey and Michael Fernandez and Francesco Gentile and Olexandr Isayev and Alexander Tropsha and Abraham C. Stern and Artem Cherkasov},
doi = {10.1038/s42256-022-00463-x},
year = {2022},
date = {2022-03-04},
urldate = {2022-03-04},
journal = {Nat Mach Intell},
volume = {4},
number = {3},
pages = {211--221},
publisher = {Springer Science and Business Media LLC},
abstract = {Deep learning has disrupted nearly every field of research, including those of direct importance to drug discovery, such as medicinal chemistry and pharmacology. This revolution has largely been attributed to the unprecedented advances in highly parallelizable graphics processing units (GPUs) and the development of GPU-enabled algorithms. In this Review, we present a comprehensive overview of historical trends and recent advances in GPU algorithms and discuss their immediate impact on the discovery of new drugs and drug targets. We also cover the state-of-the-art of deep learning architectures that have found practical applications in both early drug discovery and consequent hit-to-lead optimization stages, including the acceleration of molecular docking, the evaluation of off-target effects and the prediction of pharmacological properties. We conclude by discussing the impacts of GPU acceleration and deep learning models on the global democratization of the field of drug discovery that may lead to efficient exploration of the ever-expanding chemical universe to accelerate the discovery of novel medicines.},
keywords = {Drug Discovery, Review},
pubstate = {published},
tppubtype = {article}
}
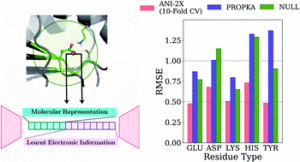
Gokcan, Hatice; Isayev, Olexandr
Prediction of protein pKawith representation learning Journal Article
In: Chem. Sci., vol. 13, no. 8, pp. 2462–2474, 2022.
Abstract | Links | BibTeX | Tags: ANI, Drug Discovery
@article{Gokcan2022,
title = {Prediction of protein p\textit{K}_{a}with representation learning},
author = {Hatice Gokcan and Olexandr Isayev},
doi = {10.1039/d1sc05610g},
year = {2022},
date = {2022-02-23},
urldate = {2022-02-23},
journal = {Chem. Sci.},
volume = {13},
number = {8},
pages = {2462--2474},
publisher = {Royal Society of Chemistry (RSC)},
abstract = {\<jats:p\>We developed new empirical ML model for protein p\<jats:italic\>K\</jats:italic\>\<jats:sub\>a\</jats:sub\>prediction with MAEs below 0.5 for all amino acid types.\</jats:p\>},
keywords = {ANI, Drug Discovery},
pubstate = {published},
tppubtype = {article}
}
2021
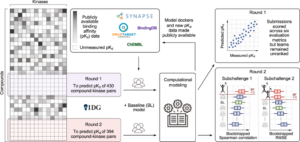
Cichońska, Anna; Ravikumar, Balaguru; Allaway, Robert J.; Wan, Fangping; Park, Sungjoon; Isayev, Olexandr; Li, Shuya; Mason, Michael; Lamb, Andrew; Tanoli, Ziaurrehman; Jeon, Minji; Kim, Sunkyu; Popova, Mariya; Capuzzi, Stephen; Zeng, Jianyang; Dang, Kristen; Koytiger, Gregory; Kang, Jaewoo; Wells, Carrow I.; Willson, Timothy M.; Tan, Mehmet; Huang, Chih-Han; Shih, Edward S. C.; Chen, Tsai-Min; Wu, Chih-Hsun; Fang, Wei-Quan; Chen, Jhih-Yu; Hwang, Ming-Jing; Wang, Xiaokang; Guebila, Marouen Ben; Shamsaei, Behrouz; Singh, Sourav; Nguyen, Thin; Karimi, Mostafa; Wu, Di; Wang, Zhangyang; Shen, Yang; Öztürk, Hakime; Ozkirimli, Elif; Özgür, Arzucan; Lim, Hansaim; Xie, Lei; Kanev, Georgi K.; Kooistra, Albert J.; Westerman, Bart A.; Terzopoulos, Panagiotis; Ntagiantas, Konstantinos; Fotis, Christos; Alexopoulos, Leonidas; Boeckaerts, Dimitri; Stock, Michiel; Baets, Bernard De; Briers, Yves; Luo, Yunan; Hu, Hailin; Peng, Jian; Dogan, Tunca; Rifaioglu, Ahmet S.; Atas, Heval; Atalay, Rengul Cetin; Atalay, Volkan; Martin, Maria J.; Jeon, Minji; Lee, Junhyun; Yun, Seongjun; Kim, Bumsoo; Chang, Buru; Turu, Gábor; Misák, Ádám; Szalai, Bence; Hunyady, László; Lienhard, Matthias; Prasse, Paul; Bachmann, Ivo; Ganzlin, Julia; Barel, Gal; Herwig, Ralf; Oršolić, Davor; Lučić, Bono; Stepanić, Višnja; Šmuc, Tomislav; Oprea, Tudor I.; Schlessinger, Avner; Drewry, David H.; Stolovitzky, Gustavo; Wennerberg, Krister; Guinney, Justin; Aittokallio, Tero
Crowdsourced mapping of unexplored target space of kinase inhibitors Journal Article
In: Nat Commun, vol. 12, pp. 3307 , 2021.
Abstract | Links | BibTeX | Tags: Drug Discovery
@article{Cicho\'{n}ska2021,
title = {Crowdsourced mapping of unexplored target space of kinase inhibitors},
author = {Anna Cicho\'{n}ska and Balaguru Ravikumar and Robert J. Allaway and Fangping Wan and Sungjoon Park and Olexandr Isayev and Shuya Li and Michael Mason and Andrew Lamb and Ziaurrehman Tanoli and Minji Jeon and Sunkyu Kim and Mariya Popova and Stephen Capuzzi and Jianyang Zeng and Kristen Dang and Gregory Koytiger and Jaewoo Kang and Carrow I. Wells and Timothy M. Willson and Mehmet Tan and Chih-Han Huang and Edward S. C. Shih and Tsai-Min Chen and Chih-Hsun Wu and Wei-Quan Fang and Jhih-Yu Chen and Ming-Jing Hwang and Xiaokang Wang and Marouen Ben Guebila and Behrouz Shamsaei and Sourav Singh and Thin Nguyen and Mostafa Karimi and Di Wu and Zhangyang Wang and Yang Shen and Hakime \"{O}zt\"{u}rk and Elif Ozkirimli and Arzucan \"{O}zg\"{u}r and Hansaim Lim and Lei Xie and Georgi K. Kanev and Albert J. Kooistra and Bart A. Westerman and Panagiotis Terzopoulos and Konstantinos Ntagiantas and Christos Fotis and Leonidas Alexopoulos and Dimitri Boeckaerts and Michiel Stock and Bernard De Baets and Yves Briers and Yunan Luo and Hailin Hu and Jian Peng and Tunca Dogan and Ahmet S. Rifaioglu and Heval Atas and Rengul Cetin Atalay and Volkan Atalay and Maria J. Martin and Minji Jeon and Junhyun Lee and Seongjun Yun and Bumsoo Kim and Buru Chang and G\'{a}bor Turu and \'{A}d\'{a}m Mis\'{a}k and Bence Szalai and L\'{a}szl\'{o} Hunyady and Matthias Lienhard and Paul Prasse and Ivo Bachmann and Julia Ganzlin and Gal Barel and Ralf Herwig and Davor Or\v{s}oli\'{c} and Bono Lu\v{c}i\'{c} and Vi\v{s}nja Stepani\'{c} and Tomislav \v{S}muc and Tudor I. Oprea and Avner Schlessinger and David H. Drewry and Gustavo Stolovitzky and Krister Wennerberg and Justin Guinney and Tero Aittokallio},
doi = {10.1038/s41467-021-23165-1},
year = {2021},
date = {2021-06-04},
urldate = {2021-06-04},
journal = {Nat Commun},
volume = {12},
pages = {3307 },
publisher = {Springer Science and Business Media LLC},
abstract = {Despite decades of intensive search for compounds that modulate the activity of particular protein targets, a large proportion of the human kinome remains as yet undrugged. Effective approaches are therefore required to map the massive space of unexplored compound\textendashkinase interactions for novel and potent activities. Here, we carry out a crowdsourced benchmarking of predictive algorithms for kinase inhibitor potencies across multiple kinase families tested on unpublished bioactivity data. We find the top-performing predictions are based on various models, including kernel learning, gradient boosting and deep learning, and their ensemble leads to a predictive accuracy exceeding that of single-dose kinase activity assays. We design experiments based on the model predictions and identify unexpected activities even for under-studied kinases, thereby accelerating experimental mapping efforts. The open-source prediction algorithms together with the bioactivities between 95 compounds and 295 kinases provide a resource for benchmarking prediction algorithms and for extending the druggable kinome.},
keywords = {Drug Discovery},
pubstate = {published},
tppubtype = {article}
}
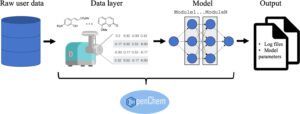
Korshunova, Maria; Ginsburg, Boris; Tropsha, Alexander; Isayev, Olexandr
OpenChem: A Deep Learning Toolkit for Computational Chemistry and Drug Design Journal Article
In: J. Chem. Inf. Model., vol. 61, no. 1, pp. 7–13, 2021, ISSN: 1549-960X.
Abstract | Links | BibTeX | Tags: Drug Discovery
@article{Korshunova2021,
title = {OpenChem: A Deep Learning Toolkit for Computational Chemistry and Drug Design},
author = {Maria Korshunova and Boris Ginsburg and Alexander Tropsha and Olexandr Isayev},
doi = {10.1021/acs.jcim.0c00971},
issn = {1549-960X},
year = {2021},
date = {2021-01-25},
urldate = {2021-01-25},
journal = {J. Chem. Inf. Model.},
volume = {61},
number = {1},
pages = {7--13},
publisher = {American Chemical Society (ACS)},
abstract = {Deep learning models have demonstrated outstanding results in many data-rich areas of research, such as computer vision and natural language processing. Currently, there is a rise of deep learning in computational chemistry and materials informatics, where deep learning could be effectively applied in modeling the relationship between chemical structures and their properties. With the immense growth of chemical and materials data, deep learning models can begin to outperform conventional machine learning techniques such as random forest, support vector machines, and nearest neighbor. Herein, we introduce OpenChem, a PyTorch-based deep learning toolkit for computational chemistry and drug design. OpenChem offers easy and fast model development, modular software design, and several data preprocessing modules. It is freely available via the GitHub repository.},
keywords = {Drug Discovery},
pubstate = {published},
tppubtype = {article}
}